How Long Does It Take for Metal to Rust?
Metal rusting is a process that can take months or years, depending on the type of metal and the conditions it’s exposed to. In this blog post, we’ll explore how long it takes for different types of metals to rust, what factors affect the rate of rusting, and how you can prevent metal from rusting.
What Is Rust Formation?
Rust formation, also known as iron oxide formation, is the process by which iron breaks down and combines with oxygen to produce a reddish-brown substance. Rust is the result of a chemical reaction between the metal surface and an oxidizing agent, such as moisture or air. When exposed to any kind of air containing these agents, metals start to corrode and break down into their constituent elements. This corrosion process weakens the structural integrity of metal objects over time.[1]
How Long Does It Take for Metal to Rust?
The time it takes for metal to rust depends on several factors, including the environmental conditions, the type of metal, and the amount of surface area exposed to moisture and oxygen. In general, a mild steel object can take anywhere from 1–5 years to show signs of rust depending on these elements.
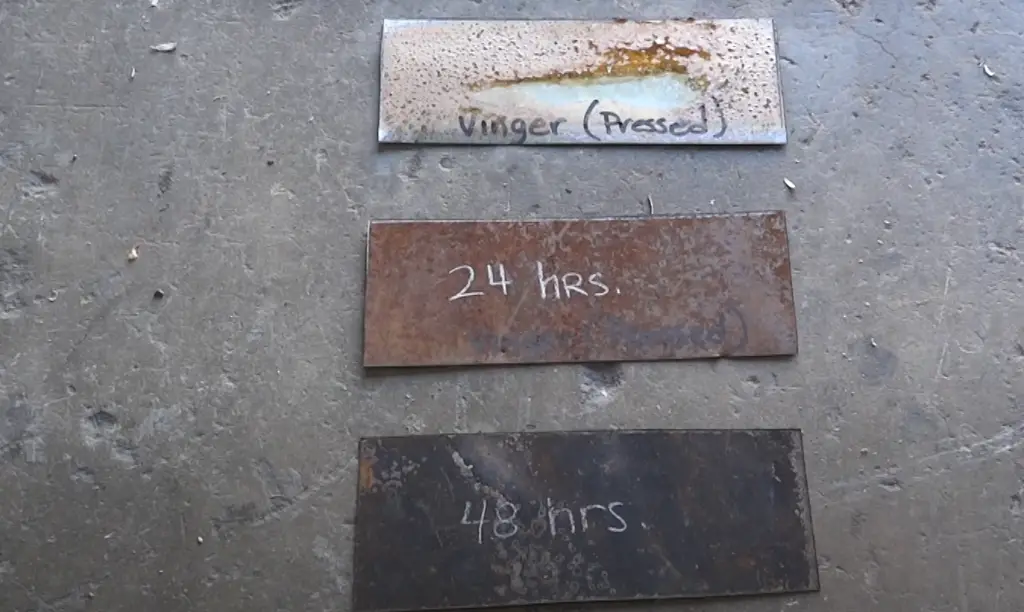
For example, when exposed to saltwater or high levels of humidity, iron objects may start to corrode in as little as 6 months. On the other hand, stainless steel is much more resistant to corrosion and may not show any obvious signs until after 10 years have passed. Aluminum is also highly resistant to corrosion and may not form any rust until after 50 years or more.
In addition to these external conditions, the amount of surface area exposed to oxygen and moisture also plays a role in how long it takes for metal to rust.
Ultimately, the best way to prevent rust is through regular maintenance and upkeep. This includes cleaning the metal surface on a regular basis and keeping it dry whenever possible. In cases where corrosion has already begun, there are a number of products available that can help to remove rust and protect the metal from further damage. [2]
The Rate of Rust Formation
Rust formation on metal surfaces depends on several factors, such as the type of metal, the environment in which it is exposed, and the amount of moisture present. For example, iron and steel are more prone to rusting than aluminum or stainless steel. In moist environments with salt or acid present (such as coastal areas or industrial sites), metals tend to corrode faster than they would in drier climates. The rate at which rust forms also depends on how much surface area of the metal is exposed to oxygen—more surface area means a quicker rate of rust formation.
The process of oxidation that occurs when metals form rust can take anywhere from minutes to years, depending on all these factors combined. Iron exposed to a wet environment without oxygen, such as underground water pipes, can corrode very slowly, whereas exposed metal in saltwater or other chemically aggressive environments can rust within hours.
In conclusion, it is difficult to give an exact answer for how long it takes for metal to rust because the process is affected by so many variables. However, with adequate protection from elements like moisture and oxygen, metals can remain corrosion-free far longer than without protection. Proper care and maintenance of metal surfaces will also help extend their life expectancy significantly. [3]
Does Saltwater Make Metal Rust Faster?
Yes, saltwater can make metal rust faster.
The chloride ions from the dissolved salts disrupt the protective oxide layer on the surface of metals, allowing oxygen to corrode them more quickly. This means that metals exposed to saltwater are more likely to experience faster rates of corrosion than those in freshwater environments.Additionally, salty air can also accelerate metal oxidation due to increased humidity levels, making it important for people living near bodies of salt water to take extra precautions when caring for their metal possessions.
To prevent rusting in a saltwater environment, it is essential to coat any exposed metal surfaces with a protective layer. Rust-resistant coatings like galvanization, epoxy paint, or powder coating can help to slow down the oxidation process and prevent rust from forming on metal surfaces in contact with saltwater. Regular maintenance of these coatings is necessary to keep them functioning properly, as saltwater can degrade them over time. Additionally, if possible it is best to avoid leaving metal items in direct contact with seawater for long periods of time. Metal objects should be rinsed off with freshwater after exposure to any kind of salty environment to reduce the risk of corrosion.
Can Saltwater Rust Steel?
Yes, saltwater can rust steel. Saltwater is an electrolyte solution that contains ions which facilitate the chemical reaction known as corrosion. Steel corrodes more quickly in salt water than it does in fresh water due to the increased conductivity of salts like sodium chloride and magnesium chloride. The higher concentrations of these ionic compounds result in greater electrochemical activity, which accelerates the rate at which steel rusts or corrodes. As a result, coastal regions with high levels of saltwater spray experience faster rates of metal corrosion than inland locations.
To protect steel from damage caused by seawater, it must be coated with a layer of protective material such as paint or galvanization.
Additionally, stainless steel grades are designed to resist corrosion better than standard carbon steel, and may be a better option when exposed to saltwater regularly. In coastal areas where salt water is present, it is important that all steel structures are properly maintained and coated with protective materials to ensure they do not succumb to corrosion. [4]
Will Salty Air Corrode Metal?
The answer to this question depends on several factors, including the type of metal in question and what other elements are present in the air. If there is a high amount of saline or salt in the air, then it can accelerate corrosion on certain metals such as aluminum, iron, and steel. As salt water falls onto these metals, it leaves behind a layer of salt that increases the corroding effect by drawing out moisture from within the metal’s surface.This makes them susceptible to rusting quickly if they are not properly protected with coatings or treatments.
It is important to protect any exposed metal surfaces from salty air if you want to preserve their life span. In addition, be aware that when working with galvanized materials near coastal areas that have higher salt content, it is important to clean them regularly and apply protective coatings such as zinc oxide. This will help prevent the metal from corroding and rusting over time.
In conclusion, salty air can accelerate corrosion on certain metals depending on several factors in the environment, but preventive measures can be taken to ensure they last longer. It is also important to note that the rate at which these metals corrode or rust depends heavily on the type of metal and other elements present in the environment.
Can Rust Occur In The Absence Of Air?
No, rust cannot occur in the absence of air.
In fact, if the metal is submerged in liquid without air exposure it will not corrode at all. So even when placed in an environment with no atmospheric oxygen and no exposure to water or other liquids, metal will not rust under these conditions.The process of corrosion only begins when oxygen combines with the molecules of the metal surface, creating an oxide layer on top of it. Without exposure to oxygen, this reaction cannot happen and hence rusting doesn’t occur. However, it’s important to note that metals can still suffer from other forms of corrosion such as pitting or cracking even without any presence of air or moisture.

Therefore, it’s important to take steps to protect metal surfaces from exposure to air and moisture if you want to reduce the chances of corrosion. This can be done by coating the metal with a protective layer, painting it, or using rust inhibitors such as oil. These measures will help extend the lifespan of your metal products and prevent them from corroding too quickly. [5]
Do Copper-nickel Alloys Provide Protection Against the Corrosive Effects of Saltwater?
Yes, copper-nickel alloys provide superior protection against the corrosive effects of saltwater. Copper-nickel alloys have a very low solubility in water, making them an ideal material for protecting metals from corrosion in marine environments. The alloy has excellent resistance to pitting and crevice corrosion when exposed to sea water and other brackish waters. Not only is it highly resistant to rust and erosion, but the alloy also exhibits good electrical conductivity which is important for many applications that require electrical connections in seawater.
All these characteristics make copper-nickel alloys a preferred choice for coastal infrastructures such as bridges, ships, marinas, yachts and others that are often exposed to harsh weather conditions. Copper-nickel alloys provide reliable protection when properly maintained, so regular inspection and maintenance of corrosion protection systems is essential for any marine structures.
Therefore, copper-nickel alloys are highly resistant to rust and corrosion from saltwater and can provide excellent long-term protection from the corrosive effects of sea water. The alloy benefits from its low solubility in water which gives it superior resistance over many other materials that may be used in similar applications. This makes copper-nickel alloys ideal for protecting metals and surfaces exposed to saltwater environments.
Electrical Conditions
The electrical conditions of the environment where metal is located will also affect how long it takes for rust to form. Metal exposed to an area with frequent high voltage discharges, such as lightning strikes, is more likely to corrode faster than metal in an area with lower current. This is because the electrical charges can cause additional oxidation and weaken the protective oxide coating on steel surfaces.
Similarly, metal objects that are part of a larger electrical circuit may be prone to accelerated corrosion due to direct electron transfer from other components in the circuit. It’s important that metal objects used in electronics are properly insulated and protected from electrical shocks.

It’s important to remember that even if your metal object does not appear visibly rusty, it can still be degrading internally due to electrical conditions. This is why it’s a good idea to regularly inspect metal objects and check for signs of rusting or corrosion. Taking proactive measures to prevent rust can help ensure that your metal items last longer.
Why Do Modern Cars Not Rust?
Modern cars do not rust as easily as older vehicles because of advances in technology. Automobile manufacturers now use galvanized steel, a zinc-coated metal, to protect car bodies from corrosion and rust. The zinc coating is bonded to the steel at the molecular level which creates an effective barrier against oxygen and moisture. This makes modern cars much more resistant to rusting than older models that used unprotected steel.
Additionally, many automakers also use a combination of synthetic or natural waxes and sealants on their cars’ exteriors which further protect them from oxidation. These protective coatings form a physical barrier between the bodywork and any potential corrosive elements including road salt and other chemicals. Modern cars are able to stay looking shiny and new thanks to these protective measures.
Despite the advancements in automotive technology, cars can still rust if not taken care of properly or exposed to extreme environmental factors. Regular car washes, waxing, and touch-ups are the best ways to ensure that a car stays rust free for as long as possible.
How Do You Keep Steel From Rusting?
Keep it dry
Moisture is the primary cause of steel corrosion, so keep your steel in a dry area and make sure any moisture that comes into contact with it is removed quickly. It’s also important to keep the steel away from acidic materials such as citric acid or vinegar.
Regularly inspect it for rust spots
Small rust spots can be treated with sandpaper, phosphoric acid, or baking soda; but if left unchecked, these spots can spread quickly across an entire piece.
Coat it with paint or oil
An oil coating will act as a barrier between metal and air and decrease its rate of oxidation. Painting over your steel is another great way to protect it against the elements.

If you choose to use this method, use a rust-proof paint that’s specifically designed for metal.
Protect it from extreme temperatures
Excessive heat or cold can cause steel to expand and contract, weakening its structural integrity and making it more vulnerable to corrosion. It’s best to keep your steel in a temperate environment where the temperature remains relatively steady.
Use zinc galvanization
Zinc galvanization is a protective coating of zinc applied to steel in order to stop it from rusting. This method is often used on outdoor structures such as fences and carports, because the zinc will protect against oxidation even with regular exposure to water or moisture. For this method, make sure your steel has been treated with an epoxy sealant before being dipped into a solution of molten zinc.
By following these tips, you can help ensure your steel remains strong and rust-free for years to come. With a bit of care and regular maintenance, you can keep your metal looking great and prevent the costly damage caused by corrosion. [6]
FAQ
Will metal rust overnight?
No, metal will not rust overnight. Many environmental factors can affect the rate of metal corrosion and oxidation. Factors such as humidity, temperature, rainfall, and air pollutants all play a role in how quickly metal will corrode. In general, it can take anywhere from several weeks to several years for metal to begin showing signs of rusting or corrosion.
How long does it take for stainless steel to rust?
Stainless steel is highly resistant to corrosion, but that resistance is not absolute. Depending on the environment and other factors, it can take anywhere from months to decades for stainless steel to start rusting or corroding.

Stainless steel’s ability to resist corrosion increases with its chromium content; higher chromium levels result in greater corrosion resistance.
What are the environmental factors that affect metal corrosion?
Humidity, temperature, rainfall, and air pollutants all play a role in how quickly metal will corrode. High levels of humidity can accelerate the rate of rusting because water helps create a reaction between oxygen and metal surfaces. Temperature also affects corrosion rate; higher temperatures increase the rate of oxidation while lower temperatures slow down the process. Additionally, air pollutants like acid rain can contribute to faster rates of rust formation. Rainfall can help flush away corrosive compounds that have formed on metal surfaces, thus slowing or preventing further corrosion or rusting.
Do different metals corrode at different rates?
Yes, different metals do corrode at different rates. The composition of the metal, its environment and other factors will affect how quickly it corrodes. Iron and steel are more prone to rusting than metals like aluminum due to their lower levels of corrosion resistance. Additionally, stainless steel is highly resistant to oxidation thanks to its chromium content. Inspecting and maintaining metal structures is important for preventing accelerated corrosion or rust formation.
What can I do to prevent metal from corroding?
Preventing corrosion on metal surfaces requires diligent inspection and maintenance. Regularly checking for signs of rust, cleaning off any built-up debris, repainting as needed and taking steps to protect against environmental conditions are all key measures one can take to slow down the rate of corrosion. In addition, using protective coatings such as zinc, paint and oil can help protect metal from corrosive elements. It is also important to use the right type of metal for your needs; some metals are more resistant to corrosion than others. For example, stainless steel is better suited to areas with high humidity or exposure to water than iron or steel. Taking these preventive measures can help extend the life of your metal structures by slowing down the rate of rust formation.
What metals rust instantly?
Most metals are vulnerable to rusting and the speed of rusting can vary depending on the environment, however certain metals are more prone to rust than others. Iron is the most common metal that rusts quickly, especially when exposed to water or air. Steel, which is an alloy of iron and other elements such as carbon, will also corrode quickly due to its high iron content. Other metals that are susceptible to corrosion include aluminum, copper, magnesium and zinc. These metals may not rust instantly but they will corrode over time if left unprotected in humid or damp environments.

To help prevent rusting of these types of metals it’s important to use protective coating such as paint or waxing.
How long does it take steel to rust away?
Steel typically takes between 5-20 years to rust away completely depending on the environment it is exposed to. If the steel is kept in a dry and protected area, or if it’s regularly maintained with paint or other protective coatings, then it can last much longer than this. In certain environments, such as coastal areas where salt water exposure is high, steel can corrode more quickly and may need to be replaced more frequently. Ultimately, how quickly metal rusts depends on many factors including its composition, whether or not it’s been treated with any protective coatings, and the environment that it’s exposed to. Understanding these elements will help you make informed decisions when selecting the right materials for your projects.
Does heat speed up rusting?
In general, heat does not make metals rust more quickly. Rusting is a chemical process that involves the metal coming into contact with water and oxygen, which are both present in the atmosphere regardless of what temperature it is. However, extreme temperatures can cause the metal to expand or contract, increasing the chances of cracks forming in the surface which can then lead to rusting.
Does rain cause rust?
The answer is yes! Rainwater contains oxygen and dissolved carbon dioxide, both of which can cause rusting. Rust forms when metal reacts with the oxygen in rainwater. The reaction causes the metal to corrode over time, leaving behind flakes of reddish-brown rust on the surface. It’s important to note, however, that not all rain will lead to rusting; areas with high concentrations of salt or other chemicals in the air will speed up corrosion and increase the likelihood it’ll happen quickly.
Useful Video: How long does steel take to rust through?
Conclusion
In conclusion, the rate at which metal rusts depends on a variety of factors – including the type of metal and the environment. Generally speaking, depending on the environment, it can take anywhere between several days to many years for metal to start showing signs of corrosion. With proper maintenance and protective coatings, however, this process can be slowed down significantly or even prevented altogether.
Ultimately, preventing corrosion is always preferable to trying to repair its effects after it has already occurred. Regular inspections and maintenance should be done in order to ensure that metals remain in good condition for as long as possible. With these procedures in place, metal surfaces can remain corrosion-free and rust-resistant for years to come.
References:
- https://strandsindustrialcoatings.com/flash-rust-and-holdtight-102/
- https://rustconverters.net/how-long-does-it-take-metal-to-rust/
- https://azrust.com/how-long-for-rust-to-form/
- https://doesitrust.com/how-long-does-it-take-for-metal-to-rust-in-saltwater/
- https://van.physics.illinois.edu/ask/listing/485
- https://capitalsteel.net/blog/why-does-steel-rust-plus-other-steel-rusting-questions-answered






